Lawesson's reagent
 | |
 | |
| Names | |
|---|---|
IUPAC name 2,4-Bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane-2,4-disulfide | |
Preferred IUPAC name 2,4-Bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane-2,4-dithione | |
| Other names Lawesson reagent; LR | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChemSpider |
|
ECHA InfoCard | 100.038.944 |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C14H14O2P2S4 |
Molar mass | 404.45 g·mol−1 |
| Appearance | Slightly yellow powder |
Melting point | 228–231 °C (442–448 °F; 501–504 K) |
Solubility in water | Insoluble |
| Hazards | |
EU classification (DSD) (outdated) | Irritant Harmful (XN) |
R-phrases (outdated) | R15/29-R20/21/22 |
S-phrases (outdated) | S7/8-S22-S45 |
| Related compounds | |
Related thiation agents | Hydrogen sulfide, Phosphorus pentasulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with P4S10.[1]
Contents
1 Preparation
2 Mechanism of action
3 Applications
4 See also
5 References
6 External links
Preparation
Lawesson's reagent is commercially available. It can also be conveniently prepared in the laboratory by heating a mixture of anisole with phosphorus pentasulfide until the mixture is clear and no more hydrogen sulfide is formed,[2] then recrystallized from toluene or xylene.
As Lawesson's reagent has a strong and unpleasant smell, it is best to prepare the compound within a fume-hood and to treat all glassware used with a decontamination solution before taking the glassware outside the fume-hood. One common and effective method of destroying the foul smelling residues is to use an excess of sodium hypochlorite (chlorine bleach).
Mechanism of action
Lawesson's reagent has a four membered ring of alternating sulfur and phosphorus atoms. With heating, the central phosphorus/sulfur four-membered ring can open to form two reactive dithiophosphine ylides (R-PS2). Much of the chemistry of Lawessons's reagent is in fact the chemistry of these reactive intermediates.
In general, the more electron rich a carbonyl is, the faster the carbonyl group will be converted into the corresponding thiocarbonyl by Lawesson's reagent.
Applications
The chemistry of Lawesson's reagent and related substances has been reviewed by several groups.[3][4][5][6] The main use of Lawesson's reagent is the thionation of carbonyl compounds. For instance, Lawesson's reagent will convert a carbonyl into a thiocarbonyl.[7] Additionally, Lawesson's reagent has been used to thionate enones, esters,[8]lactones,[9]amides, lactams,[10] and quinones.
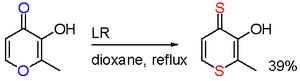
In one study, reaction of maltol with LR results in a selective oxygen replacement in two positions.[11]
A combination of silver perchlorate and Lawesson's reagent is able to act as an oxophilic Lewis acid with the ability to catalyze the Diels-Alder reaction of dienes with α,β-unsaturated aldehydes.
Alcohols may be converted to thiols by treatment with Lawesson's reagent.[12]
Lawesson's reagent reacts with sulfoxides to form a thio product, which are then desulfurized to form thioethers.[citation needed] Therefore, it can be used as a reducing agent for sulfoxide.
See also
- Woollins' reagent
References
^ Lecher, H. Z.; Greenwood, R. A.; Whitehouse, K. C.; Chao, T. H. (1956). "The Phosphonation of Aromatic Compounds with Phosphorus Pentasulfide". J. Am. Chem. Soc. 78 (19): 5018. doi:10.1021/ja01600a058..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Thomsen, I.; Clausen, K.; Scheibye, S.; Lawesson, S.-O. (1990). "Thiation with 2,4-Bis(4-methoxyphenyl)-1,3,2,4-Dithiadiphosphetane 2,4-disulfide: N-Methylthiopyrrolidone". Organic Syntheses.CS1 maint: Multiple names: authors list (link) ; Collective Volume, 7, p. 372
^ Cherkasov, R. A.; Kutyrev, G. A.; Pudovik, A. N. (1985). "Tetrahedron report number 186 Organothiophosphorus reagents in organic synthesis". Tetrahedron (Review). 41 (41): 2567. doi:10.1016/S0040-4020(01)96363-X.
^ Foreman, M.S.; Woollins, J.D. (2000). "Organo-P-S and P-Se heterocycles". J. Chem. Soc., Dalton Trans. (10): 1533–1543. doi:10.1039/b000620n.
^ Martin Jesberger; Thomas P. Davis; Leonie Barner (2003). "Applications of Lawesson's Reagent in Organic and Organometallic Syntheses". Synthesis (Review). 2003 (13): 1929–1958. doi:10.1055/s-2003-41447.
^ Cava, M. P.; Levinson, M. I. (1985). "Thionation reactions of Lawesson's reagents". Tetrahedron. 41 (22): 5061–5087. doi:10.1016/S0040-4020(01)96753-5.
^ Pedersen, B. S.; Scheibye, S.; Nilsson, N. H.; Lawesson, S.-O. (1978). Bull. Soc. Chim. Belg. (87): 223. Missing or empty|title=(help)CS1 maint: Multiple names: authors list (link)
^ Jones, B. A.; Bradshaw, J. S. (1984). "Synthesis and reduction of thiocarboxylic O-esters". Chem. Rev. (Review). 84 (84): 17. doi:10.1021/cr00059a002.
^ Scheibye, S.; Kristensen, J.; Lawesson, S.-O. (1979). "Studies on organophosphorus compounds—XXVII Synthesis of thiono-, thiolo- and dithiolactones". Tetrahedron. 35 (35): 1339–1343. doi:10.1016/0040-4020(79)85027-9.CS1 maint: Multiple names: authors list (link)
^ Shabana, R.; Scheibye, S.; Clausen, K.; Olesen, S. O.; Lawesson, S.-O. (1980). Nouveau Journal de Chimie. 1980 (4): 47. Missing or empty|title=(help)CS1 maint: Multiple names: authors list (link)
^ Brayton, D.; Jacobsen, F. E.; Cohen, S. M.; Farmer, P. J. (2006). "A novel heterocyclic atom exchange reaction with Lawesson's reagent: a one-pot synthesis of dithiomaltol". Chemical Communications. 2006 (2): 206–208. doi:10.1039/b511966a. PMID 16372107.
^ Nishio, Takehiko (1989). "A novel transformation of alcohols to thiols". Journal of the Chemical Society, Chemical Communications. 1989 (4): 205–206. doi:10.1039/C39890000205.
External links
"Lawesson's Reagent". Organic Chemistry Portal. Retrieved 2007-10-16.