Alcohol by volume

The alcohol by volume shown on a bottle of absinthe.
Alcohol by volume (abbreviated as ABV, abv, or alc/vol) is a standard measure of how much alcohol (ethanol) is contained in a given volume of an alcoholic beverage (expressed as a volume percent).[1][2][3] It is defined as the number of millilitres (mL) of pure ethanol present in 100 mL of solution at 20 °C (68 °F). The number of millilitres of pure ethanol is the mass of the ethanol divided by its density at 20 °C, which is 0.78924 g/mL. The ABV standard is used worldwide. The International Organization of Legal Metrology has tables of density of water–ethanol mixtures at different concentrations and temperatures.
In some countries, e.g., France, alcohol by volume is often referred to as degrees Gay-Lussac (after the French chemist Joseph Louis Gay-Lussac),[4] although there is a slight difference since the Gay-Lussac convention uses the International Standard Atmosphere value for temperature, 15 °C (59 °F).
Contents
1 Volume change
2 Typical levels
3 Alcohol proof
4 Alcohol by weight
5 Prediction of alcohol content
5.1 Wine
5.2 Beer
6 See also
7 Notes
8 References
9 Bibliography
10 External links
Volume change
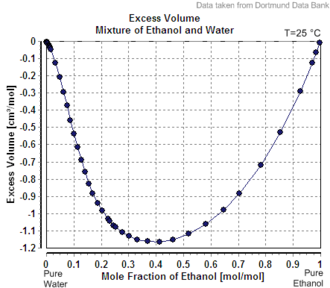
Change in volume with increasing ABV
Mixing two solutions of alcohol of different strengths usually causes a change in volume. Mixing pure water with a solution less than 24% by mass causes a slight increase in total volume, whereas the mixing of two solutions above 24% causes a decrease in volume.[a] The phenomenon of volume changes due to mixing dissimilar solutions is called "partial molar volume". Water and ethanol are both polar solvents. When water is added to ethanol, the smaller water molecules are attracted to the ethanol's hydroxyl group, and each molecule alters the polarity field of the other. The attraction allows for closer spacing between molecules than is usually found in non-polar mixtures.
Thus, ABV is not the same as volume fraction expressed as a percentage. Volume fraction, which is widely used in chemistry, is defined as the volume of a particular component divided by the sum of all components in the mixture when they are measured separately. To make a 50% v/v ethanol solution, for example, you would measure 50 mL of ethanol and separately measure 50 mL of water, then mix the two together. The resulting volume of solution will likely not measure 100 mL due to the change of volume on mixing.
Typical levels
Details about typical amounts of alcohol contained in various beverages can be found in the articles about them.
| Drink | Typical ABV |
|---|---|
Fruit juice (naturally occurring) | 0%-0.09% |
| Low-alcohol beer | 0.05%–1.2% |
| Kvass | 0.05%–1.5% |
| Kefir | 0.2%–2.0% |
| Kombucha | 0.5%–1.5% |
| Boza | 1.0% |
| Chicha | 1.0%–11% (usually 1%–6%) |
| Beer | 2.0%–12% (usually 4%–6%) |
| Cider | 2.0%–12% (usually 4%–8%) |
| Alcopops | 4.0%–17.5% |
| Malt liquor | 5.0%+ |
| Makgeolli | 6.5%–7% |
Barley wine (strong ale) | 8%–15% |
| Mead | 8%–16% |
| Wine | 9%–16% (most often 12.5%–14.5%)[5] |
| Kilju | 15%–17% |
| Dessert wine | 14%–25% |
| Sake | 15% (or 18%–20% if not diluted prior to bottling) |
| Liqueurs | 15%–55% |
| Fortified wine | 15.5%–20%[6] (in the European Union, 18%–22%) |
| Soju | 17%–45% (usually 19%) |
| Shochu | 25%–45% (usually 25%) |
| Rượu đế | 27%-45% (usually 35%- except Ruou tam -40-45%) |
| Bitters | 28%–45% |
| Applejack | 30%-40% |
Mezcal, Tequila | 32%–60% (usually 40%) |
| Vodka | 35%–95% (usually 40%, minimum of 37.5% in the European Union) |
| Rum | 37.5%–80% (usually 40%) |
| Brandy | 35%–60% (usually 40%) |
| Grappa | 37.5%–60% |
| Ouzo | 37.5%+ |
| Pálinka | 37.5%–86% (usually 52%) |
| Cachaça | 38%–54% |
| Sotol | 38%–60% |
| Stroh | 38%–80% |
| Fernet | 39%–45% |
| Nalewka | 40%–45% |
| Tsipouro | 40%-45% |
| Gin | 37.5%–50% |
| Scotch whisky | 40%–63.5% |
| Whisky | 40%–68% (usually 40%, 43% or 46%) |
| Baijiu | 40%–65% |
| Chacha | 40%–70% |
| Răchie (Central/Southeast European drink) | 42%–86% |
| Maotai | 43%-53% |
| Absinthe | 45%–89.9% |
| Țuică (Romanian drink) | 30%–65% (usually 35%–55%) |
| Arak | 60%–65% |
| Oghi | 60%-75% |
| Poitín | 60%–95% |
| Centerbe (herb liqueur) | 70% |
| Neutral grain spirit | 85%–95% |
| Cocoroco | 93%–96%[citation needed] |
| Rectified spirit | 95% up to a practical limit of 95.6% |
Alcohol proof
Another way of specifying the amount of alcohol is alcohol proof, which in the United States is twice the alcohol-by-volume number, while in the United Kingdom it is 1.75 times the number (expressed as a percentage).[7][8] For example, 40% abv is 80 proof in the US and 70 proof in the UK. However, since 1980, alcohol proof in the UK has been replaced by abv as a measure of alcohol content.
Alcohol by weight
In the United States, a few states regulate and tax alcoholic beverages according to alcohol by weight (ABW), expressed as a percentage of total mass. Some brewers print the ABW (rather than the ABV) on beer containers, particularly on low-point versions of popular domestic beer brands.
One can use the following equation to convert between ABV and ABW:
- ABV×0.78924=ABW×density of beverage at 20 C in g/mL{displaystyle ABVtimes 0.78924=ABWtimes {text{density of beverage at 20 C in g/mL}}}
At relatively low ABV, the alcohol percentage by weight is about 4/5 of the ABV (e.g., 3.2% ABW is about 4% ABV).[9] However, because of the miscibility of alcohol and water, the conversion factor is not constant but rather depends upon the concentration of alcohol. 100% ABW is equivalent to 100% ABV.
Prediction of alcohol content
During the production of wine and beer, yeast is added to a sugary solution. During fermentation, the yeasts consume the sugars and produce alcohol. The density of sugar in water is greater than the density of alcohol in water. A hydrometer is used to measure the change in specific gravity (SG) of the solution before and after fermentation. The volume of alcohol in the solution can then be estimated. There are a number of empirical formulae which brewers and winemakers use to estimate the alcohol content of the liquor made.
Wine
The simplest method for wine has been described by English author C.J.J. Berry:[8]
- ABV=(Starting SG−Final SG)/7.36{displaystyle ABV=(mathrm {Starting~SG} -mathrm {Final~SG} )/7.36}
Beer
The calculation for beer is:[10]
ABV=133.62×(Starting SG−Final SG){displaystyle ABV=133.62times left(mathrm {Starting~SG} -mathrm {Final~SG} right)}
However, many brewers use the following formula which uses a different constant (different probably due to temperature variations and rounding):[11]
ABV=131.25(Starting SG−Final SG){displaystyle ABV=131.25left(mathrm {Starting~SG} -mathrm {Final~SG} right)}
It is derived in this manner:[10]
- ABV=ρC2H5OHρCO2×ρH2OρC2H5OH×ρCO2ρH2O×100{displaystyle ABV={frac {rho _{C2H5OH}}{rho _{CO2}}}times {frac {rho _{H2O}}{rho _{C2H5OH}}}times {frac {rho _{CO2}}{rho _{H2O}}}times 100}
- ABV=ρC2H5OHρCO2×ρH2OρC2H5OH×ρOG−ρFGρH2O×100{displaystyle ABV={frac {rho _{C2H5OH}}{rho _{CO2}}}times {frac {rho _{H2O}}{rho _{C2H5OH}}}times {frac {rho _{OG}-rho _{FG}}{rho _{H2O}}}times 100}
- ABV=ρC2H5OHρCO2×ρH2OρC2H5OH×(Starting SG−Final SG)×100{displaystyle ABV={frac {rho _{C2H5OH}}{rho _{CO2}}}times {frac {rho _{H2O}}{rho _{C2H5OH}}}times left(mathrm {Starting~SG} -mathrm {Final~SG} right)times 100}
- ABV=46.068444.0095×1.00.78934(Starting SG−Final SG)×100{displaystyle ABV={frac {46.0684}{44.0095}}times {frac {1.0}{0.78934}}left(mathrm {Starting~SG} -mathrm {Final~SG} right)times 100}
- ABV=1.046780.78934×(Starting SG−Final SG)×100{displaystyle ABV={frac {1.04678}{0.78934}}times left(mathrm {Starting~SG} -mathrm {Final~SG} right)times 100}
- ABV=133.62×(Starting SG−Final SG){displaystyle ABV=133.62times left(mathrm {Starting~SG} -mathrm {Final~SG} right)}
1.05 is the ratio (by mass) of ethanol molecules produced for every molecule of CO2 produced[dubious ] (46.07 g/mol C2H6O / 44.01 g/mol CO2 = 1.0468) from a single molecule of glucose. The number 0.7936 is the specific gravity of a 100% ethanol solution[12]. Both are unit-less measurements. The difference between the starting SG and the final SG measures the specific gravity lost to CO2 release.
For higher ABV above 6% many brewers use this formula:[13]
- ABV=1.050.79(Starting SG−Final SGFinal SG)×100{displaystyle ABV={frac {1.05}{0.79}}left({frac {mathrm {Starting~SG} -mathrm {Final~SG} }{mathrm {Final~SG} }}right)times 100}
See also
- Alcohol proof
- Unit of alcohol
- Standard drink
- Apparent molar property
- Excess molar quantity
- Volume fraction
Notes
^ See data in the CRC Handbook of Chemistry and Physics, 49th edition, pp. D-151 and D-152. Mixing a solution above 24% with a solution below 24% may cause an increase or a decrease, depending on the details.
References
^ "Lafayette Brewing Co". www.lafayettebrewingco.com. Archived from the original on 2012-02-19. Retrieved 2012-02-04..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "Glossary of whisky and distillation". www.celtic-whisky.com. Archived from the original on 2012-02-12. Retrieved 2012-02-04.
^ "English Ales Brewery Monterey British Brewing Glossary". www.englishalesbrewery.com. Archived from the original on 2012-02-19. Retrieved 2012-02-04.
^ "Joseph Louis Gay-Lussac (1778–1850)". chemistry.about.com. Retrieved 2008-07-05.
^ Robinson 2006, p. 10.
^ Robinson 2006, p. 279.
^ Regan 2003.
^ ab Berry 1998.
^ "Alcohol Content In Beer". www.realbeer.com. Archived from the original on 4 July 2008. Retrieved 2008-07-05.
^ ab "Calculate Percent Alcohol in Beer". BrewMoreBeer.com. Retrieved 2014-07-03.
^ "Get to Know Your Alcohol (By Volume)". BeerAdvocate.com. Retrieved 2014-07-03.
^ "S.G. Table for Ethanol-Water". www.separationprocesses.com. Retrieved 2016-08-31.
^ Anon, 2012, Industrial Microbiology Beer Fermentation Practical, School Of Applied Sciences, RMIT University, Melbourne
Bibliography
.mw-parser-output .refbegin{font-size:90%;margin-bottom:0.5em}.mw-parser-output .refbegin-hanging-indents>ul{list-style-type:none;margin-left:0}.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>dd{margin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none}.mw-parser-output .refbegin-100{font-size:100%}
Hehner, Otto (1880). Alcohol Tables: giving for all specific gravities, from 1.0000 to 0.7938, the percentages of absolute alcohol, by weight and volume. London: J & A Churchill. ASIN B0008B5HOU.
Berry, C. J. J. (1998). First Steps in Winemaking. Nexus Special Interests. ISBN 978-1-85486-139-9.
Regan, Gary (2003). The Joy of Mixology. Clarkson Potter. ISBN 978-0-609-60884-5.
Robinson, Jancis (2006). The Oxford Companion to Wine (3rd ed.). Oxford: OUP. ISBN 978-0-19-860990-2.
External links
"How do brewers measure the alcohol in beer?". HowStuffWorks. 12 December 2000.
Jayes, Wayne. "Alcohol Strength and Density". sugartech.co.za. The Sugar Engineers.









