Catecholamine

Catechol

dopamine

norepinephrine (noradrenaline)
epinephrine (adrenaline)
A catecholamine (/ˌkætəˈkoʊləmiːn/; CA) is a monoamine, an organic compound that has a catechol (benzene with two hydroxyl side groups at carbons 1 and 2) and a side-chain amine.[1]
Catechol can be either a free molecule or a substituent of a larger molecule, where it represents a 1,2-dihydroxybenzene group.
Catecholamines are derived from the amino acid tyrosine, which is derived from dietary sources as well as synthesis from phenylalanine.[2] Catecholamines are water-soluble and are 50%-bound to plasma proteins in circulation.
Included among catecholamines are epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine. Release of the hormones epinephrine and norepinephrine from the adrenal medulla of the adrenal glands is part of the fight-or-flight response.[3]
Tyrosine is created from phenylalanine by hydroxylation by the enzyme phenylalanine hydroxylase. Tyrosine is also ingested directly from dietary protein. Catecholamine-secreting cells use several reactions to convert tyrosine serially to L-DOPA and then to dopamine. Depending on the cell type, dopamine may be further converted to norepinephrine or even further converted to epinephrine.[4]
Various stimulant drugs (e.g., a number of substituted amphetamines) are catecholamine analogues.
Contents
1 Structure
2 Production and degradation
2.1 Location
2.2 Biosynthesis
2.3 Degradation
3 Function
3.1 Modality
3.2 Effects
3.3 Function in plants
4 See also
5 References
6 External links
Structure
Catecholamines have the distinct structure of a benzene ring with two hydroxyl groups, an intermediate ethyl chain, and a terminal amine group.
Phenylethanolamines such as norepinephrine have a hydroxyl group on the ethyl chain.
Production and degradation
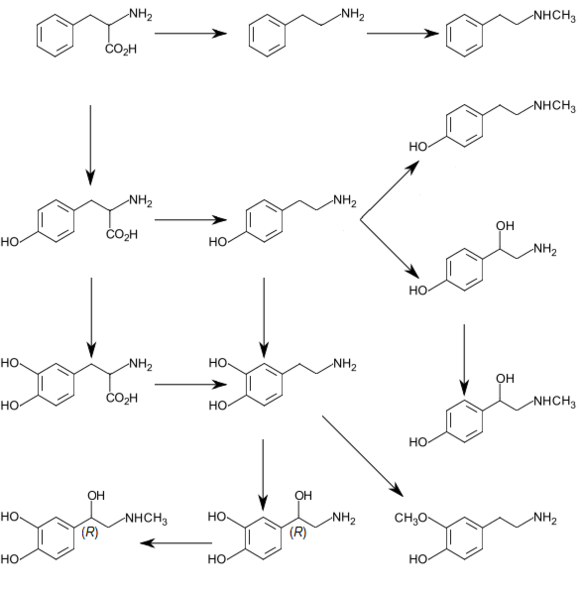
Biosynthetic pathways for catecholamines and trace amines in the human brain[5][6][7] L-Phenylalanine is converted into L-tyrosine by an aromatic amino acid hydroxylase (AAAH) enzyme (phenylalanine 4-hydroxylase), with molecular oxygen (O2) and tetrahydrobiopterin as cofactors. L-Tyrosine is converted into L-DOPA by another AAAH enzyme (tyrosine 3-hydroxylase) with tetrahydrobiopterin, O2, and ferrous iron (Fe2+) as cofactors. L-DOPA is converted into dopamine by the enzyme aromatic L-amino acid decarboxylase (AADC), with pyridoxal phosphate as the cofactor. Dopamine itself is also used as precursor in the synthesis of the neurotransmitters norepinephrine and epinephrine. Dopamine is converted into norepinephrine by the enzyme dopamine β-hydroxylase (DBH), with O2 and L-ascorbic acid as cofactors. Norepinephrine is converted into epinephrine by the enzyme phenylethanolamine N-methyltransferase (PNMT) with S-adenosyl-L-methionine as the cofactor. |
Location
Catecholamines are produced mainly by the chromaffin cells of the adrenal medulla and the postganglionic fibers of the sympathetic nervous system. Dopamine, which acts as a neurotransmitter in the central nervous system, is largely produced in neuronal cell bodies in two areas of the brainstem: the ventral tegmental area and the substantia nigra, the latter of which contains neuromelanin-pigmented neurons. The similarly neuromelanin-pigmented cell bodies of the locus ceruleus produce norepinephrine. Epinephrine is produced in small groups of neurons in the human brain which express its synthesizing enzyme, phenylethanolamine N-methyltransferase;[8] these neurons project from a nucleus that is adjacent (ventrolateral) to the area postrema and from a nucleus in the dorsal region of the solitary tract.[8]
Biosynthesis
Dopamine is the first catecholamine synthesized from DOPA. In turn, norepinephrine and epinephrine are derived from further metabolic modification of dopamine. The enzyme dopamine hydroxylase requires copper as a cofactor (not shown in the diagram) and DOPA decarboxylase requires PLP (not shown in the diagram). The rate limiting step in catecholamine biosynthesis through the predominant metabolic pathway is the hydroxylation of L-tyrosine to L-DOPA.
Catecholamine synthesis is inhibited by alpha-methyl-p-tyrosine (AMPT), which inhibits tyrosine hydroxylase.[citation needed]
The amino acids phenylalanine and tyrosine are
precursors for catecholamines. Both amino acids are
found in high concentrations in the plasma and brain.
In mammals, tyrosine can be formed from dietary phenylalanine
by the enzyme phenylalanine hydroxylase, found in large amounts in the liver. Insufficient
amounts of phenylalanine hydroxylase result in
phenylketonuria, a metabolic disorder that leads
to intellectual deficits unless treated by dietary
manipulation.
Catecholamine synthesis usually is considered to
begin with tyrosine. The enzyme tyrosine hydroxylase
(TH) converts the amino acid l-tyrosine into 3,4-
dihydroxyphenylalanine (l-DOPA). The hydroxylation
of l-tyrosine by TH results in the formation of
the DA precursor l-DOPA, which is metabolized by
l-aromatic amino acid decarboxylase (AADC; see
Cooper et al., 2002) to the transmitter dopamine. This step occurs so rapidly that it is difficult to measure l-DOPA in the brain without first inhibiting AADC. In neurons that use DA as the transmitter, the decarboxylation of l-DOPA to DA is the final step in transmitter, However, in those neurons using norepinephrine (also known as noradrenaline) or epinephrine (adrenaline) as transmitters, the enzyme dopamine b-hydroxylase (DBH), which converts DA to yield NE, is also present. In still other neurons in which epinephrine is the transmitter, a third enzyme phenylethanolamine N-methyltransferase, PNMT) converts NE into Epi. Thus, a cell that uses Epi as its transmitter contains four enzymes (TH, AADC, DBH, and PNMT), whereas NE neurons contain only three enzymes (lacking PNMT) and DA cells only two (TH and AADC).
Degradation
Catecholamines have a half-life of a few minutes when circulating in the blood. They can be degraded either by methylation by catechol-O-methyltransferases (COMT) or by deamination by monoamine oxidases (MAO).
MAOIs bind to MAO, thereby preventing it from breaking down catecholamines and other monoamines.
Catabolism of catecholamines is mediated via two main enzymes: catechol-o-methyltransferase (COMT) which is present in the synaptic cleft and cytosol of the cell and monoamine oxidase (MAO) which is located in the mitochondrial membrane. Both enzymes require cofactors: COMT uses Mg2+ as a cofactor while MAO uses FAD. The first step of the catabolic process is mediated by either MAO or COMT which depends on the tissue and location of catecholamines (for example degradation of catecholamines in the cleft is mediated by COMT because MAO is a mitochondrial enzyme). The next catabolic steps in the pathway involve alcohol dehydrogenase, aldehyde dehydrogenase and aldehyde reductase. The end product of epinephrine and norepinephrine is VMA (vanillylmandelic acid) which is excreted in the urine. Dopamine catabolism leads to the production of HVA (homovanillic acid).[9]
Function
Modality
Two catecholamines, norepinephrine and dopamine, act as neuromodulators in the central nervous system and as hormones in the blood circulation. The catecholamine norepinephrine is a neuromodulator of the peripheral sympathetic nervous system but is also present in the blood (mostly through "spillover" from the synapses of the sympathetic system).
High catecholamine levels in blood are associated with stress, which can be induced from psychological reactions or environmental stressors such as elevated sound levels, intense light, or low blood sugar levels.
Extremely high levels of catecholamines (also known as catecholamine toxicity) can occur in central nervous system trauma due to stimulation and/or damage of nuclei in the brainstem, in particular those nuclei affecting the sympathetic nervous system. In emergency medicine, this occurrence is widely known as catecholamine dump.
Extremely high levels of catecholamine can also be caused by neuroendocrine tumors in the adrenal medulla, a treatable condition known as pheochromocytoma.
High levels of catecholamines can also be caused by monoamine oxidase A (MAO-A) deficiency. As MAO-A is one of the enzymes responsible for degradation of these neurotransmitters, its deficiency increases the bioavailability of these neurotransmitters considerably. It occurs in the absence of pheochromocytoma, neuroendocrine tumors, and carcinoid syndrome, but it looks similar to carcinoid syndrome such as facial flushing and aggression.[10][11]
The acute porphyria's can cause elevated catecholamines.[12]
Effects
Catecholamines cause general physiological changes that prepare the body for physical activity (fight-or-flight response). Some typical effects are increases in heart rate, blood pressure, blood glucose levels, and a general reaction of the sympathetic nervous system. Some drugs, like tolcapone (a central COMT-inhibitor), raise the levels of all the catecholamines.
Catecholamine is secreted into urine after being broken down, and its secretion level can be measured for the diagnosis of illnesses associated with catecholamine levels in the body.[13]Urine testing for catecholamine is used to detect pheochromocytoma.
Function in plants
"They have been found in 44 plant families, but no essential metabolic function has been established for them. They are precursors of benzo[c]phenanthridine alkaloids, which are the active principal ingredients of many medicinal plant extracts. CAs have been implicated to have a possible protective role against insect predators, injuries, and nitrogen detoxification. They have been shown to promote plant tissue growth, somatic embryogenesis from in vitro cultures, and flowering. CAs inhibit indole-3-acetic acid oxidation and enhance ethylene biosynthesis. They have also been shown to enhance synergistically various effects of gibberellins."[14]
See also
- Catechol-O-methyl transferase
- Catecholaminergic polymorphic ventricular tachycardia
- History of catecholamine research
- Hormone
- Julius Axelrod
- Peptide hormone
- Phenethylamines
- Steroid hormone
- Sympathomimetics
- Vanillylmandelic acid
References
^ Fitzgerald, P. A. (2011). "Chapter 11. Adrenal Medulla and Paraganglia". In Gardner, D. G.; Shoback, D. Greenspan’s Basic & Clinical Endocrinology (9th ed.). New York: McGraw-Hill. Retrieved October 26, 2011..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Purves, D.; Augustine, G. J.; Fitzpatrick, D.; Hall, W. C.; LaMantia, A. S.; McNamara, J. O.; White, L. E., eds. (2008). Neuroscience (4th ed.). Sinauer Associates. pp. 137&ndash, 8. ISBN 978-0-87893-697-7.
^ "Catecholamines". Health Library. San Diego: University of California. Archived from the original on 2011-07-16.
^ Joh, T. H.; Hwang, O. (1987). "Dopamine Beta-Hydroxylase: Biochemistry and Molecular Biology". Annals of the New York Academy of Sciences. 493: 342–350. doi:10.1111/j.1749-6632.1987.tb27217.x. PMID 3473965.
^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". Eur. J. Pharmacol. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
^ ab Kitahama K, Pearson J, Denoroy L, Kopp N, Ulrich J, Maeda T, Jouvet M (1985). "Adrenergic neurons in human brain demonstrated by immunohistochemistry with antibodies to phenylethanolamine-N-methyltransferase (PNMT): discovery of a new group in the nucleus tractus solitarius". Neurosci. Lett. 53 (3): 303–308. doi:10.1016/0304-3940(85)90555-5. PMID 3885079.
^ Eisenhofer, G.; Kopin, IJ; Goldstein, DS (2004). "Catecholamine metabolism: a contemporary view with implications for physiology and medicine". Pharmacol. Rev. 3 (56): 331–49. PMID 15317907.
^ Manor, I.; Tyano, S.; Mel, E.; Eisenberg, J.; Bachner-Melman, R.; Kotler, M.; Ebstein, R. P. (2002). "Family-Based and Association Studies of Monoamine Oxidase A and Attention Deficit Hyperactivity Disorder (ADHD): Preferential Transmission of the Long Promoter-Region Repeat and its Association with Impaired Performance on a Continuous Performance Test (TOVA)". Molecular Psychiatry. 7 (6): 626–632. doi:10.1038/sj.mp.4001037. PMID 12140786.
^ Brunner, H. G. (1996). "MAOA Deficiency and Abnormal Behaviour: Perspectives on an Association". Ciba Foundation Symposium. 194: 155–164, discussion 164–167. PMID 8862875.
^ Stewart MF, Croft J, Reed P, New JP. "Acute intermittent porphyria and phaeochromocytoma: shared features". J Clin Pathol. 60: 935–6. doi:10.1136/jcp.2005.032722. PMC 1994495. PMID 17660335.
^ "Catecholamines in Urine". webmd.com. Retrieved 4 May 2017.
^ Kuklin, A. I.; Conger, B. V. (1995). "Catecholamines in Plants". Journal of Plant Growth Regulation. 14 (2): 91–97. doi:10.1007/BF00203119.
External links
Catecholamines at the US National Library of Medicine Medical Subject Headings (MeSH)