Electric potential
| electric potential | |
|---|---|
Common symbols | V |
| SI unit | volt |
Other units | statvolt |
| In SI base units | V = kg m2 A−1 s−3 |
Extensive? | yes |
| Dimension | M L2T−3I−1 |
An electric potential (also called the electric field potential, potential drop or the electrostatic potential) is the amount of work needed to move a unit of positive charge from a reference point to a specific point inside the field without producing an acceleration. Typically, the reference point is the Earth or a point at infinity, although any point beyond the influence of the electric field charge can be used.
According to classical electrostatics, electric potential is a scalar quantity denoted by V or occasionally φ,[1] equal to the electric potential energy of any charged particle at any location (measured in joules) divided by the charge of that particle (measured in coulombs). By dividing out the charge on the particle a quotient is obtained that is a property of the electric field itself.
This value can be calculated in either a static (time-invariant) or a dynamic (varying with time) electric field at a specific time in units of joules per coulomb (J C−1), or volts (V). The electric potential at infinity is assumed to be zero.
In electrodynamics, when time-varying fields are present, the electric field cannot be expressed only in terms of a scalar potential. Instead, the electric field can be expressed in terms of both the scalar electric potential and the magnetic vector potential.[2] The electric potential and the magnetic vector potential together form a four vector, so that the two kinds of potential are mixed under Lorentz transformations.
Contents
1 Introduction
2 Electrostatics
2.1 Electric potential due to a point charge
3 Generalization to electrodynamics
4 Units
5 Galvani potential versus electrochemical potential
6 See also
7 References
8 Further reading
Introduction
Classical mechanics explores concepts such as force, energy, potential etc. Force and potential energy are directly related. A net force acting on any object will cause it to accelerate. As an object moves in the direction in which the force accelerates it, its potential energy decreases: the gravitational potential energy of a cannonball at the top of a hill is greater than at the base of the hill. As it rolls downhill its potential energy decreases, being translated to motion, kinetic energy.
It is possible to define the potential of certain force fields so that the potential energy of an object in that field depends only on the position of the object with respect to the field. Two such force fields are the gravitational field and an electric field (in the absence of time-varying magnetic fields). Such fields must affect objects due to the intrinsic properties of the object (e.g., mass or charge) and the position of the object.
Objects may possess a property known as electric charge and an electric field exerts a force on charged objects. If the charged object has a positive charge the force will be in the direction of the electric field vector at that point while if the charge is negative the force will be in the opposite direction. The magnitude of the force is given by the quantity of the charge multiplied by the magnitude of the electric field vector.
Electrostatics
The electric potential at a point r in a static electric field E is given by the line integral
VE=−∫CE⋅dℓ{displaystyle V_{mathbf {E} }=-int _{C}mathbf {E} cdot mathrm {d} {boldsymbol {ell }},}
where C is an arbitrary path connecting the point with zero potential to r. When the curl ∇ × E is zero, the line integral above does not depend on the specific path C chosen but only on its endpoints. In this case, the electric field is conservative and determined by the gradient of the potential:
E=−∇VE.{displaystyle mathbf {E} =-mathbf {nabla } V_{mathbf {E} }.,}
Then, by Gauss's law, the potential satisfies Poisson's equation:
- ∇⋅E=∇⋅(−∇VE)=−∇2VE=ρ/ε0,{displaystyle mathbf {nabla } cdot mathbf {E} =mathbf {nabla } cdot left(-mathbf {nabla } V_{mathbf {E} }right)=-nabla ^{2}V_{mathbf {E} }=rho /varepsilon _{0},,}
where ρ is the total charge density (including bound charge) and ∇· denotes the divergence.
The concept of electric potential is closely linked with potential energy. A test charge q has an electric potential energy UE given by
- UE=qV.{displaystyle U_{mathbf {E} }=q,V.,}
The potential energy and hence also the electric potential is only defined up to an additive constant: one must arbitrarily choose a position where the potential energy and the electric potential are zero.
These equations cannot be used if the curl ∇ × E ≠ 0, i.e., in the case of a non-conservative electric field (caused by a changing magnetic field; see Maxwell's equations). The generalization of electric potential to this case is described below.
Electric potential due to a point charge
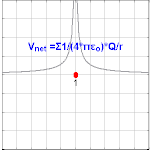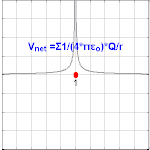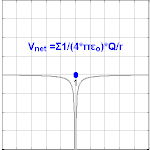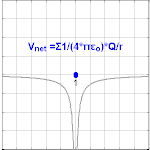
The electric potential created by a charge Q is V=Q/(4πεor). Different values of Q will make different values of electric potential V (shown in the image).
The electric potential arising from a point charge Q, at a distance r from the charge is observed to be
- VE=14πε0Qr,{displaystyle V_{mathbf {E} }={frac {1}{4pi varepsilon _{0}}}{frac {Q}{r}},,}
where ε0 is the permittivity of vacuum.[3]VE{displaystyle V_{mathbf {E} }}
The electric potential for a system of point charges is equal to the sum of the point charges' individual potentials. This fact simplifies calculations significantly, because addition of potential (scalar) fields is much easier than addition of the electric (vector) fields.
The equation given above for the electric potential (and all the equations used here) are in the forms required by SI units. In some other (less common) systems of units, such as CGS-Gaussian, many of these equations would be altered.
Generalization to electrodynamics
When time-varying magnetic fields are present (which is true whenever there are time-varying electric fields and vice versa), it is not possible to describe the electric field simply in terms of a scalar potential V because the electric field is no longer conservative: ∫CE⋅dℓ{displaystyle textstyle int _{C}mathbf {E} cdot mathrm {d} {boldsymbol {ell }}}

Instead, one can still define a scalar potential by also including the magnetic vector potential A. In particular, A is defined to satisfy:
- B=∇×A,{displaystyle mathbf {B} =mathbf {nabla } times mathbf {A} ,,}
where B is the magnetic field. Because the divergence of the magnetic field is always zero due to the absence of magnetic monopoles, such an A can always be found. Given this, the quantity
- F=E+∂A∂t{displaystyle mathbf {F} =mathbf {E} +{frac {partial mathbf {A} }{partial t}}}
is a conservative field by Faraday's law and one can therefore write
- E=−∇V−∂A∂t,{displaystyle mathbf {E} =-mathbf {nabla } V-{frac {partial mathbf {A} }{partial t}},,}
where V is the scalar potential defined by the conservative field F.
The electrostatic potential is simply the special case of this definition where A is time-invariant. On the other hand, for time-varying fields,
- −∫abE⋅dℓ≠V(b)−V(a),{displaystyle -int _{a}^{b}mathbf {E} cdot mathrm {d} {boldsymbol {ell }}neq V_{(b)}-V_{(a)},,}
unlike electrostatics.
Units
The SI derived unit of electric potential is the volt (in honor of Alessandro Volta), which is why a difference in electric potential between two points is known as voltage. Older units are rarely used today. Variants of the centimeter gram second system of units included a number of different units for electric potential, including the abvolt and the statvolt.
Galvani potential versus electrochemical potential
Inside metals (and other solids and liquids), the energy of an electron is affected not only by the electric potential, but also by the specific atomic environment that it is in. When a voltmeter is connected between two different types of metal, it measures not the electric potential difference, but instead the potential difference corrected for the different atomic environments.[4] The quantity measured by a voltmeter is called electrochemical potential or fermi level, while the pure unadjusted electric potential V is sometimes called Galvani potential ϕ{displaystyle phi }
See also
- Absolute electrode potential
- Electrochemical potential
- Electrode potential
References
^ Goldstein, Herbert (June 1959). Classical Mechanics. United States: Addison-Wesley. p. 383. ISBN 0201025108..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Griffiths, David J. Introduction to Electrodynamics. Pearson Prentice Hall. pp. 416–417. ISBN 978-81-203-1601-0.
^ "CODATA Value: electric constant". The NIST Reference on Constants, Units, and Uncertainty. US National Institute of Standards and Technology. June 2015. Retrieved 2015-09-25.2014 CODATA recommended values
^ Bagotskii VS (2006). Fundamentals of electrochemistry. p. 22. ISBN 978-0-471-70058-6.
Further reading
.mw-parser-output .refbegin{font-size:90%;margin-bottom:0.5em}.mw-parser-output .refbegin-hanging-indents>ul{list-style-type:none;margin-left:0}.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>dd{margin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none}.mw-parser-output .refbegin-100{font-size:100%}
Politzer P, Truhlar DG (1981). Chemical Applications of Atomic and Molecular Electrostatic Potentials: Reactivity, Structure, Scattering, and Energetics of Organic, Inorganic, and Biological Systems. Boston, MA: Springer US. ISBN 978-1-4757-9634-6.
Sen K, Murray JS (1996). Molecular Electrostatic Potentials: Concepts and Applications. Amsterdam: Elsevier. ISBN 978-0-444-82353-3.
Griffiths DJ (1998). Introduction to Electrodynamics (3rd. ed.). Prentice Hall. ISBN 0-13-805326-X.
Jackson JD (1999). Classical Electrodynamics (3rd. ed.). USA: John Wiley & Sons, Inc. ISBN 978-0-471-30932-1.
Wangsness RK (1986). Electromagnetic Fields (2nd., Revised, illustrated ed.). Wiley. ISBN 978-0-471-81186-2.
| Wikimedia Commons has media related to Electric potential. |








